Graphite and AMC

In recent years, many companies have joined the race to develop the next graphite mine. With the rapid development of alternative energy sources for many applications, improved batteries with increased storage capacity have become critical to world growth. As a result, the lithium, cadmium, nickel, vanadium, and graphite markets have seen an increase in demand; especially high purity, large sized graphite flakes, which are able to attract a significant premium in the current market.
According to Allied Market Research, the graphite market was valued at $14.3 billion in 2019, and is expected to reach a total market value of US$21.6 billion by 2027. This is driven by high demand for lithium-ion batteries and through graphite electrode, electric arc furnace steel production.
Clearly, graphite mining and processing will feature significantly in the future. Individuals and firms like AMC Consultants (AMC) with substantial experience in graphite projects, from exploration, resource and reserve estimation through to mining and processing, will be in demand. Graphite deposits have unique characteristics for mining, as well as in geotechnical performance, processing and tailings management, which makes graphite experience essential for technical studies and JORC or NI 43-101 reporting.
About Graphite
Graphite is a form of elemental carbon, the other two being coal and diamond. It has a black to steel grey colour. It is extremely soft and feels greasy to touch in its natural form. It is opaque, even in the finest particles. Graphite is a good conductor of heat and electricity. It can stand temperatures up to 3,000oC in an inert atmosphere, though in the presence of oxygen it burns between 620oC and 720oC. It is unaffected by most acids and reagents but yields graphitic acid on treatment with a mixture of potassium nitrate and nitric acid. Graphite has the highest natural strength and stiffness of any material currently known.

Source Allied Market Research
Most people are surprised to learn that a lithium-ion battery contains 20 to 30 times more graphite than lithium. These batteries are becoming increasingly popular in power tools and electric scooters. Growth will be further accelerated with increased use in hybrid and fully electric passenger and utility vehicles. Graphite also finds extensive use in nuclear reactor fuel cells, solar power storage systems and electronics ranging from smartphones to laptops. It has been categorized as a critical, strategic mineral by several governments, including the United States and governments in the European Union. Other uses include crucibles for melting non-ferrous metals, lubricants, foundry facing, pencils, protective coatings for wood, construction materials in the aircraft industry, and large industrial applications such as the electrodes in electric furnaces.
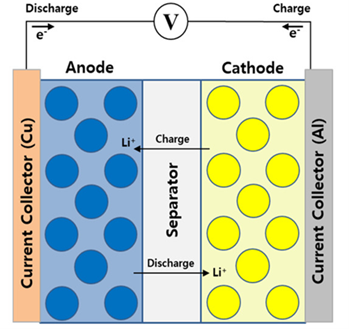
A simplified lithium battery with spherical graphite in blue and lithium oxides in yellow
(www.mdpi.com)
Graphite is a form of elemental carbon, the other two being coal and diamond. It has a black to steel grey colour. It is extremely soft and feels greasy to touch in its natural form. It is opaque, even in the finest particles. Graphite is a good conductor of heat and electricity. It can stand temperatures up to 3,000oC in an inert atmosphere, though in the presence of oxygen it burns between 620oC and 720oC. It is unaffected by most acids and reagents but yields graphitic acid on treatment with a mixture of potassium nitrate and nitric acid. Graphite has the highest natural strength and stiffness of any material currently known.
Both natural and synthetic graphite are available to industry. There are three distinct types of natural graphite, being amorphous graphite, flake graphite and high crystalline graphite.
Graphite types
Amorphous graphite
This is the most abundant form of graphite and has a relatively low carbon content of between 70% and 80%. There is no visible crystallinity, and it is not of a suitable quality for most applications.

Flake graphite
Flake graphite
Flake graphite is a less common form of graphite, with a carbon range of 85% to 98%. It is priced approximately four times higher than amorphous graphite and is used in many traditional applications. These include lubrication additives and furnace electrodes, where the graphite is introduced as a paste that is continuously fed into the top of the electrode while it burns away at the other end inside the furnace.
Large flakes are desirable for many of the emerging technology applications such as Li-ion battery anode material, and as a result the larger flake sizes tend to attract a high price.
High crystalline graphite
This is also referred to as vein, lump, or crystalline vein graphite; and it is currently only extracted in Sri Lanka. The carbon content of high crystalline graphite ranges from 90% to 99%. Due to its scarcity and high costs, the viability of using this type of graphite in most industrial applications is limited.

High crystalline graphite

Synthetic graphite
Synthetic graphite
Synthetic graphite is a manufactured product made by high-temperature treatment of amorphous carbon materials. In the United States, the primary feedstocks used for making synthetic graphite are calcined petroleum coke and coal tar pitch. This makes it up to ten times more expensive to produce than natural graphite, and as a result, less appealing for use in most applications.
What is Graphene?
In recent years, graphene has generated a large amount of interest due to its very unique properties. It is a natural material that is the basic building block of graphite, achieved when the thickness is reduced to less than 10 atoms. Although it was “discovered” in the 1940s, it took until 2004 before scientists figured out how to isolate it from graphite particles, using what is termed a “scotch tape” exfoliation method. Since then, there has been tremendous interest in graphene, with research scientists demonstrating its suitability for combination with a vast range of materials to greatly enhance the performance of those materials. There has also been an explosion in the number of patents being taken out, as industry has been preparing for the start of the new and deeply disruptive “graphene age”. The table below lists the key properties of graphene that industry is seeking to employ.
Graphene
| Property | Comment |
|---|---|
| Thinnest material | It can be as thin as only one carbon atom in thickness i.e. only ~0.345 nm thick |
| Stronger than steel | It is one of the hardest materials in the world, being harder than diamonds and 200 times stronger than steel (1,100TPa/125 GPa) of the same thickness, but it is very flexible and will not break. As an example, a graphene sheet 1 m2 in size could support a 4 kg cat, but that sheet would weigh only as much as the cat’s whiskers. |
| Optical properties | One atom thick layer sheets absorb ~2.3% visible light, making it transparent. |
| Light and stretchable | It weighs only 0.77 milligrams per square meter and is stretchable up to 20% of its initial length. It has the largest surface area to volume ratio of any material. |
| Impermeable | It is completely impermeable. Even helium atoms cannot pass through it. |
| Thermal conductivity | It is a perfect thermal conductor (over 5,000 W/mK), giving it five times the conductivity of graphite. It conducts heat in all directions i.e. it is an isotropic conductor. |
| Electronic properties | It has the highest electrical current density (one million times that of copper), and the highest intrinsic mobility (100 times that of silicon). It has a lower resistivity than any other known material at room temperature. |
| Chemical properties | It is an inert material and does not readily react with other atoms. However, it can “absorb” different atoms and molecules, leading to changes in its properties. It can be functionalized by several different chemical groups, resulting in different materials such as graphene oxide and fluorinated graphene. |
| Other qualities | It is self–repairing. Graphene can self-repair holes in its sheets when exposed to molecules containing carbon. |
| Reactive | It is the most reactive form of carbon. |
Source: The Investors’ Guide to Graphene
Graphene can be manufactured from different carbon sources and is not limited to graphite as a source material, with manufacturing methods including:
- Carbon vapour deposition (CVD).
- Carbon dioxide reduction.
- Epitaxial growth on silicon carbides and metal substrates.
- Graphite oxide reduction.
- Graphite sonication.
- Carbon nanotubes (CNT).
- Mechanical exfoliation.
Graphite Deposits
Graphite is of metamorphic origin and usually found as veins, lenses, pockets, and as thin laminae disseminated in gneisses, schists and phyllites. It is usually associated with minerals such as quartz, calcite, micas, tourmalines, and with iron-rich meteorites. Flake graphite, the most valuable form, is found disseminated in metamorphosed quartz-mica-feldspar gneiss, schist and marble. High crystalline graphite is precipitated during propylitic hydrothermal alteration of the volcanic host rock. Amorphous graphite is formed by metamorphosis of coal seams through magmatic intrusions. Amorphous graphite is soft, black, earthy, and has less lustre than crystalline graphite.
Commercially viable grades can vary from approximately 8% to more than 20% of total graphitic carbon (TGC) in the deposit, with cut-off grades usually between 2% and 5% TGC.
Overall, more than 100 known deposits exist, and most countries have at least one graphite deposit. However, world production is mainly focussed in Austria, Brazil, Canada, China, Germany, India, Madagascar, Sri Lanka, Sweden, Mozambique, and Zimbabwe.
Typical process flowsheet
Graphite is of metamorphic origin and usually found as veins, lenses, pockets, and as thin laminae disseminated in gneisses, schists and phyllites. It is usually associated with minerals such as quartz, calcite, micas, tourmalines, and with iron-rich meteorites. Flake graphite, the most valuable form, is found disseminated in metamorphosed quartz-mica-feldspar gneiss, schist and marble. High crystalline graphite is precipitated during propylitic hydrothermal alteration of the volcanic host rock. Amorphous graphite is formed by metamorphosis of coal seams through magmatic intrusions. Amorphous graphite is soft, black, earthy, and has less lustre than crystalline graphite.
Commercially viable grades can vary from approximately 8% to more than 20% of total graphitic carbon (TGC) in the deposit, with cut-off grades usually between 2% and 5% TGC.
Overall, more than 100 known deposits exist, and most countries have at least one graphite deposit. However, world production is mainly focussed in Austria, Brazil, Canada, China, Germany, India, Madagascar, Sri Lanka, Sweden, Mozambique, and Zimbabwe.
Figure 1. Typical graphite process flowsheet
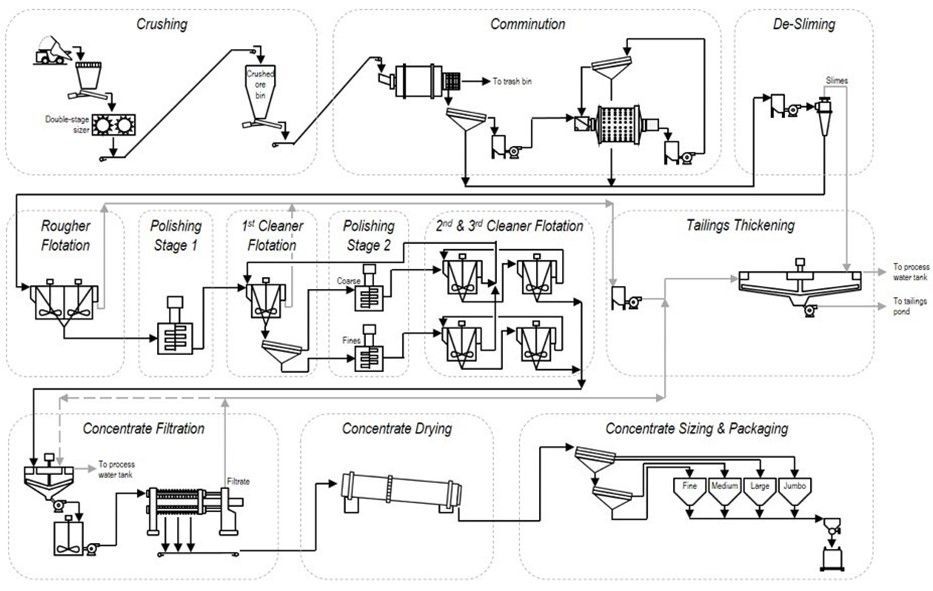
Source: SRG Graphite
Graphite is a naturally hydrophobic mineral and was reputed to be the first mineral concentrated by flotation. The flotation separation from feldspar, quartz, mica, and marble gangue is enhanced by the addition of small amounts of a neutral oil such as kerosene, and by the addition of a frother such as pine oil or an alcohol.
Cleaning of the rougher concentrate, even after repeated gentle, low solid content regrinding with pebble mills in between cleaner flotation stages is challenging because graphite smears easily, which could render remaining gangue particles floatable. Also, some graphite flakes have a siliceous skeleton, or are composed of a flaky layer of mica in between layers of flaky graphite, making it difficult to obtain an ultra-high-grade product. Using flotation and multiple cleaning stages, a grade of up to 97% to 98% TGC can be achieved. In order to obtain ultra-high-purity flake graphite with up to 99.98% TGC for the nuclear industry, chemical digestion and removal of impurities by leaching is necessary.
Products
Final products sold by the operations can be tailored to demand, depending on the initial flake size, purity, and effort that the producer is prepared to expend before shipping the graphite concentrate to market.
The most important initial differentiator is obviously purity. However, once certain purity objectives have been achieved, e.g., TGC > 97%, size specifications are the most important differentiator. Typical operations would screen the product into three to five different size fractions, with bagging facilities allowing the blending of size fractions for certain customers, if required. In addition, fines can be formed into briquettes, which can still achieve a reasonable price.
A high-premium product is spherical graphite, which is produced by polishing the edges of large flakes, producing high-purity, spherical particles with larger overall surface area and better conductivity, providing enhanced battery anode characteristics. Spherical graphite is produced mainly in China, with high production costs elsewhere preventingmost other producers from entering this high-value market. Figure 2 shows the basic steps of spherical-graphite processing to create a high-value final product. However, a significant additional cost is incurred due to loss of product volume through the generation of fines during the additional processing steps.
Figure 2. Flake to spherical graphite – the process
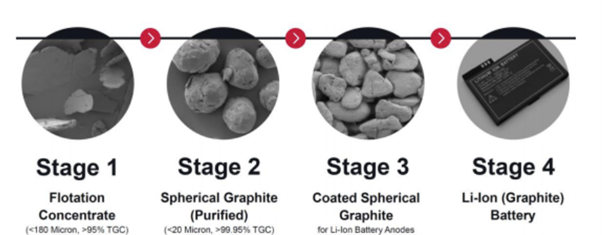
Source: Peninsula Mines / Finfeed.com
AMC’s capability
Many graphite deposits are under active evaluation or development, and this will continue as a response to rising demand, especially in the battery market, but also in the development of alternative uses for graphite-based materials such as graphene.
AMC’s graphite capability is backed by specific practical and technical experience, as well as a global reputation in all types of commodities in all phases of the mining cycle. This includes resource estimation, feasibility studies, project development, underground and open-pit mine design, and processing. AMC understands the critical factors that are unique to graphite, including flake size, purity and market sensitivities, technical and practical challenges and risks, and their management and solutions.
Need Help?
Meet some of our graphene experts
Mark Burnett
Principal Geologist
-
Short bio
Mark has more than 25 years of experience in the mining industry and has extensive exposure to the mining-value chain, including early-stage exploration projects, shaft sinking, operational mines, mergers, acquisitions, and asset disposals. Mark joined AMC in 2018 as a Principal Geologist, where his primary responsibilities are undertaking and managing technical geological works, including mineral resource estimation, technical reporting, and client support in relation to production and exploration advice.
Tracie Burrows
Advisory Lead - Geometallurgy
-
Short bio
Tracie’s experience includes resource estimation, feasibility studies, technical audits and reviews, due diligence studies, and independent technical specialist reports. She has project management experience in geological pre-feasibility and feasibility studies, as well as mine closure and commissioning. Tracie can act as a Competent Person for reporting in accordance with the JORC Code, with significant experience in porphyry copper deposits, base metals, hard rock tin, and gold.
As of October 2020, Tracie is a representative of the Australian Institute of Geoscientists (AIG) on the Joint Ore Reserves Committee (JORC).
Meet some of our graphene experts
Rob Chesher
Technical Manager – Business Development
-
Short bio
Robert’s expertise is in corporate and technical (metallurgical) consulting, focusing on operational and performance reviews, improvements, and optimization. In his various career roles, he has been involved in studies and reviews at all levels in copper, gold, platinum, coal, nickel, and other base metals. He has a record of achieving significant and sustainable operational improvements across the mining, oil and gas, and manufacturing sectors. Robert has worked on Russian gold projects extensively in the last few years including Peschanka, Olimpiada, Verninskoye, Kuranakh, and Chertovo Koryto.
Paul Greenhill
Principal Consultant
-
Short bio
Paul’s primary expertise is mineral processing and pyrometallurgy applied to feasibility study evaluation, techno-economic modeling/evaluation, technical due diligence and business improvement. Paul’s experience includes eight years with Comalco in bauxite metallurgy for its Weipa operations and smelting technology for its smelting operations, due diligence and operational consulting for CSM EL Toqui Pb/Zn mine in southern Chile. Paul has completed due diligence and feasibility studies for Goondicum mineral sands project, Qld, the Mt Carrington Au/Ag project near Drake, NSW for White Rock Minerals Limited, the scoping study of the Haggan Uranium deposit, Sweden for Aura Energy, Ozernoe Lead and Zinc project, Russia, Wonawinta Silver project, NSW, El Toqui Zinc mine, Chile, Broula Magnetite mine, NSW.
References:
W Grigor; The Investors’ Guide to Graphene; Far East Capital Limited; February 2015
M R Patil, K.S. Shivakurnar, S. Prakash and R. Bhima Rao; Estimation of the liberation size of graphite in a schistose rock and its response to beneficiation; Minerals and Mining; November 1997
I H Redeker, E H Bentzen; Plant and Laboratory Practice in Non-metallic Mineral Flotation; Colorado School of Mines Research Institute; Undated
S Moores; Spherical graphite: how is it made?; Industrial Minerals; August 2013
Subscribe for the latest news & events
Contact Details
Useful Links
News & Insights




